GIFT Jiayu Wan Team Publishes Breakthroughs in Rational Design of Solid-State Electrolytes in Science Advances and Advanced Materials
The research team led by Associate Professor Jiayu Wan from Global Institute of Future Technology, SJTU has recently published groundbreaking articles in Science Advances and Advanced Materials, focusing on the ultrafast synthesis design of solid-state electrolytes and interfacial design of high entropy solid state electrolyte. These studies were conducted in collaboration with teams from Westlake University (Yizhou Zhu and Yao Yang) and Southern University of Science and Technology (Lin Zeng).
The first study, titled "Unveiling Phase Evolution of Complex Oxides toward Precise Solid-State Synthesis," was published in Science Advances (https://www.science.org/doi/10.1126/sciadv.adx3927). Solid-state synthesis is one of the most widely used methods for preparing inorganic materials due to its simplicity and scalability. However, the reaction pathways for complex multi-component oxides are often unpredictable, resulting in the presence of impurities that are thermodynamically stable but functionally inefficient, which severely impact material performances and reproducibility. Current synthesis methods lack control over intermediate phases and heavily rely on “trial-and-error" adjustments, which uses empirical knowledge to select precursor ratios and thermal treatments, leading to low efficiency and limited success rates. Recent studies suggest that intermediates not only serve as transitional species but can also kinetically dictate the nucleation pathways of final products. Thus, the key challenge lies in "controllable construction" of specific intermediates to steer reactions toward desired phases, particularly for high-performance electrolytes and complex oxides.
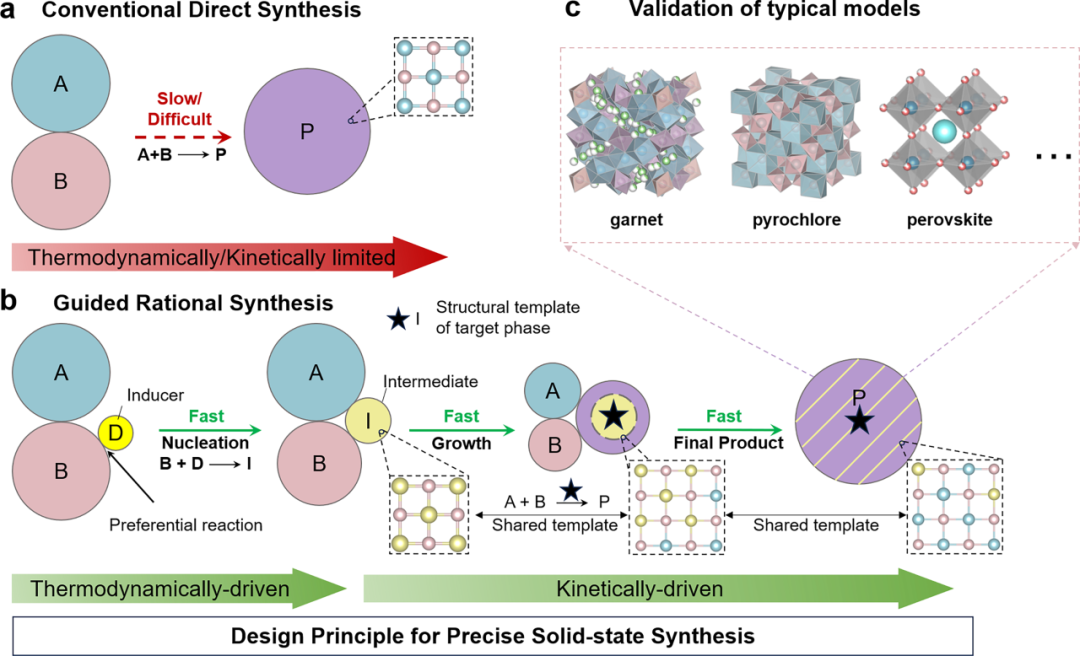
Figure 1. Schematic of the proposed guiding principle for the precise synthesis of complex solids.
To address this challenge, the team of Jiayu Wan, in collaboration with the team of Zhu Yizhou, proposed an inducer-facilitated assembly structural templating (i-FAST) strategy. By introducing trace inducers to preferentially form intermediates that are structurally similar to the target materials, the team established well-defined reaction pathways, enabling controllable synthesis of complex oxides. The i-FAST strategy was validated for ultrafast synthesis of three distinct oxides: garnet, pyrochlore and perovskite, demonstrating its broad applicability. The structural templating approach bypasses traditional "black-box synthesis" limitations by leveraging thermodynamically predesigned intermediates to guide rapid, high-purity nucleation of target materials.
Notably, the team developed a quasi-in situ XRD characterization technique coupled with ultrafast synthesis, effectively decoupling acquisition time and data resolution to capture and analyze key intermediate phases at sub-second timescales. Combined with multi-scale TEM analysis and first-principles calculations, the study systematically revealed the pivotal role of structural templates in directing kinetic pathways and enhancing reaction selectivity, offering a new paradigm for rational design and efficient synthesis of solid-state materials.
The second study, titled "Rational Design of High-Entropy Garnet Electrolytes via Computational Screening for Stable Lithium Interfaces in All-Solid-State Batteries," was published in Advanced Materials. This work challenges the conventional assumption of lithium-metal compatibility in garnet solid-state electrolytes (SSEs) by demonstrating that high-entropy LLZO (HE LLZO) systems exhibit composition-dependent interfacial stability. Through systematic investigation of Zr-site substituents, the paper proposes that thermodynamic reducibility-quantified via reaction energies between Li rich oxides (LixMyOz) with lithium metal-serve as a critical descriptor for predicting interfacial reactivity. High-risk dopants (e.g., Nb, Mo, W, Cr, Bi) prone to lithium reactions were screened, leading to the successful synthesis of a novel interfacial-stable HE-LLZO (Li6.6La3Zr0.4Sn0.4Hf0.4Sc0.2Ta0.6O12). The study established a critical design principle for high-entropy SSEs: cation selection must simultaneously satisfy phase stability, ionic mobility and thermodynamic immunity to lithium-driven reduction. This work provides a generalizable framework for designing high-entropy SSEs and multi-element materials, paving the way for next-generation solid-state batteries. (https://doi.org/10.1002/adma.202509838)
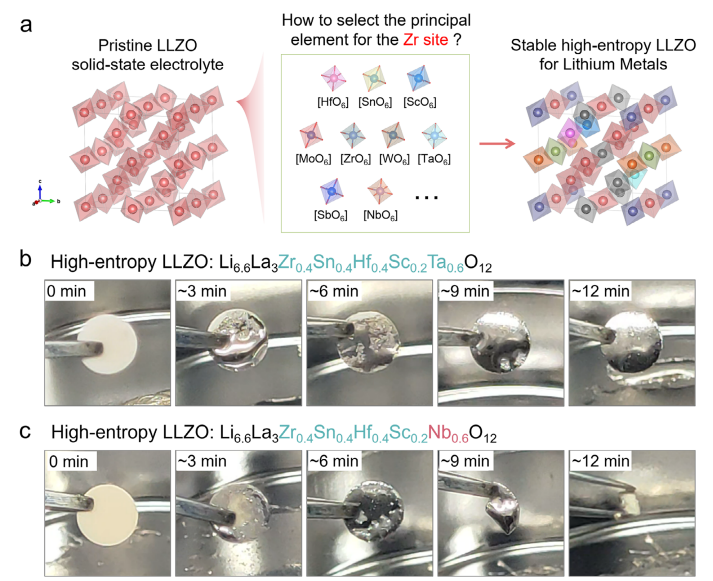
Figure 2. Typical stable and unstable HE-LLZO SSEs in contact with lithium metal.
Corresponding Author Profile

Jiayu Wan
Associate Professor, Doctoral Supervisor and Executive Director of the Future Battery Research Center at Global Institute of Future Technology, SJTU. Prof Wan finished postdoctoral research at Stanford University under Professors Yi Cui and Zhenan Bao, earned his Ph.D. at the University of Maryland under Professor Liangbing Hu (now Chair Professor at Yale University), and holds a B.S. from Huazhong University of Science and Technology. His research focuses on electrochemical energy storage devices and advanced manufacturing. In recent years, he has published over 100 SCI papers in leading journals such as Science, Nature Nanotechnology, Nature Energy, Science Advances and Joule, with 15,000+ citations and an h-index of 57. His work has been featured by international media outlets. Honors include the Dorothy M. and Earl S. Hoffman Award (one recipient globally each year), China Scholarship Council Award for Outstanding Self-Financed Students Abroad, Clarivate Highly Cited Researcher (2024), and World’s Top 2% Scientists (2020-present). He was also named among Forbes China’s 100 Outstanding Overseas Returnees, Shanghai S&T 35, and received the ACS Energy & Fuels Rising Star Award, RSC JMCA and Green Chemistry Emerging Investigator Awards and Springer Nature Med-X Young Scientist Award. He serves as a Youth Editorial Board Member for eScience, National Science Open, Materials Today Energy, Chinese Chemical Letters, Information & Functional Materials, Chain, Carbon Energy, and has delivered 100+ invited talks at international conferences and institutions.
Deep Energy Lab Website: https://www.x-mol.com/groups/deepenergy




